I started thinking about what other subjects could be more easily taught with a 3D model present. I came up with a few ideas that I still plan to try, but the one that stuck out was metallic crystal structures (which might have been influenced by finding out that Byriah Loper published a book last year).
Some important concepts in chemistry are the unit cells of the three basic metallic crystal structures: simple cubic (or primitive cubic), body-centred cubic and face-centred cubic (or cubic close-packed). Among other things, chemistry students are generally asked to identify the number of nearest neighbour atoms to each (equivalent) atom. Instead of the usual dot/sphere to represent an atom, I decided to make a wireframe structure where the nearest neighbours are all connected. By folding each of these connectors from the same size of paper, I could also show the difference in size of the unit cells.
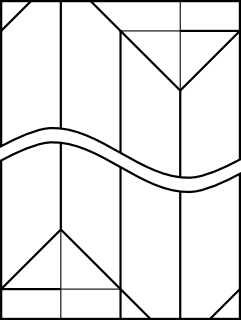
The first model was the simple cubic structure. I decided to fold each connector from a 2:1 rectangle, similar to the chemistry model kit. In order to join together three units for a cubic frame, I needed a bit of extra paper as shown by the black outline labelled A

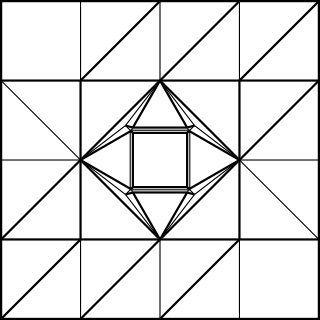
That extra 1/4 of the paper width was used to make the flap, as shown in the CP
Joining 12 units together gives you this:

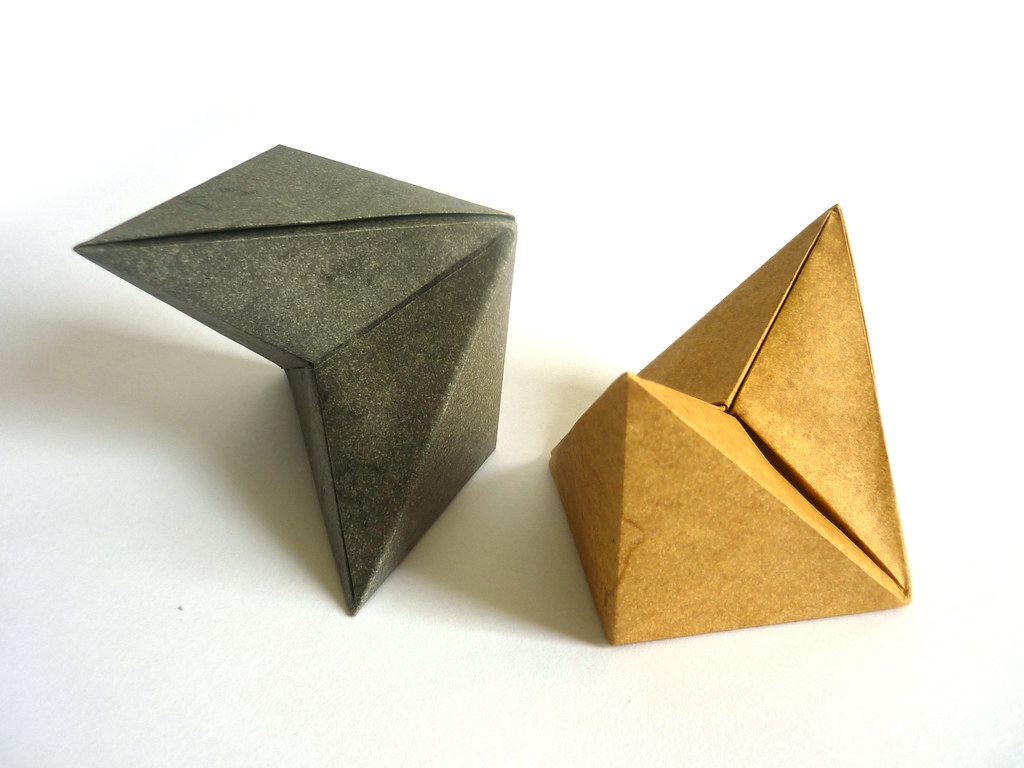
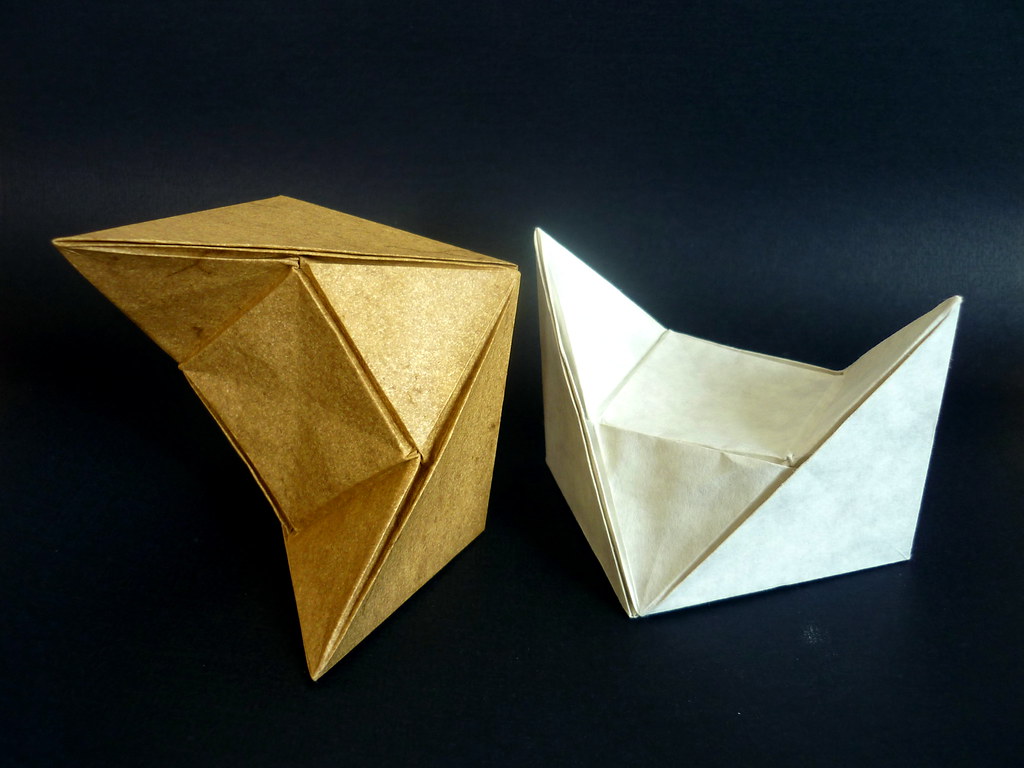
The simple cubic structure is naturally just a cube. For the other structures, I needed a larger cubic frame to represent the bounds of the unit cell in addition to the connector pieces. Following a bit of math, the frame of a body-centred cell is 2/√3 times the length of the connectors. The connector (A) and the frame piece (B) look like this:

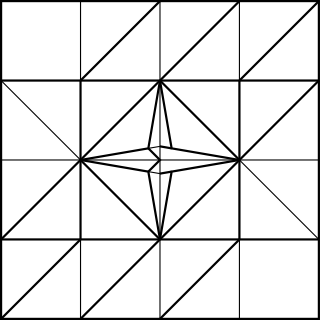
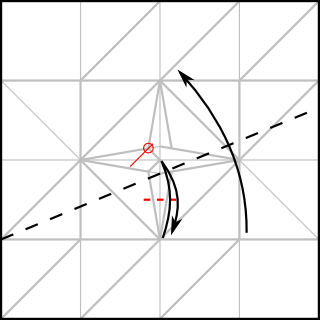
Note that I added 1/4 of the paper width again for the cubic frame. The outer frame is folded just like the simple cubic structure, whereas the connectors have to be folded to join at an arccos(1/√3) angle. The following CP achieves this goal:
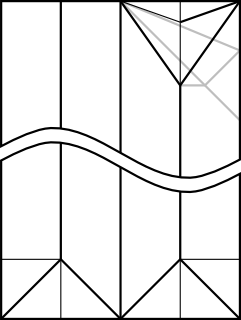
The unit only connects on one side, while the other side just hold the unit's shape. Ideally, the non-connecting end should be folded with an angle slightly greater than 45°, such as:

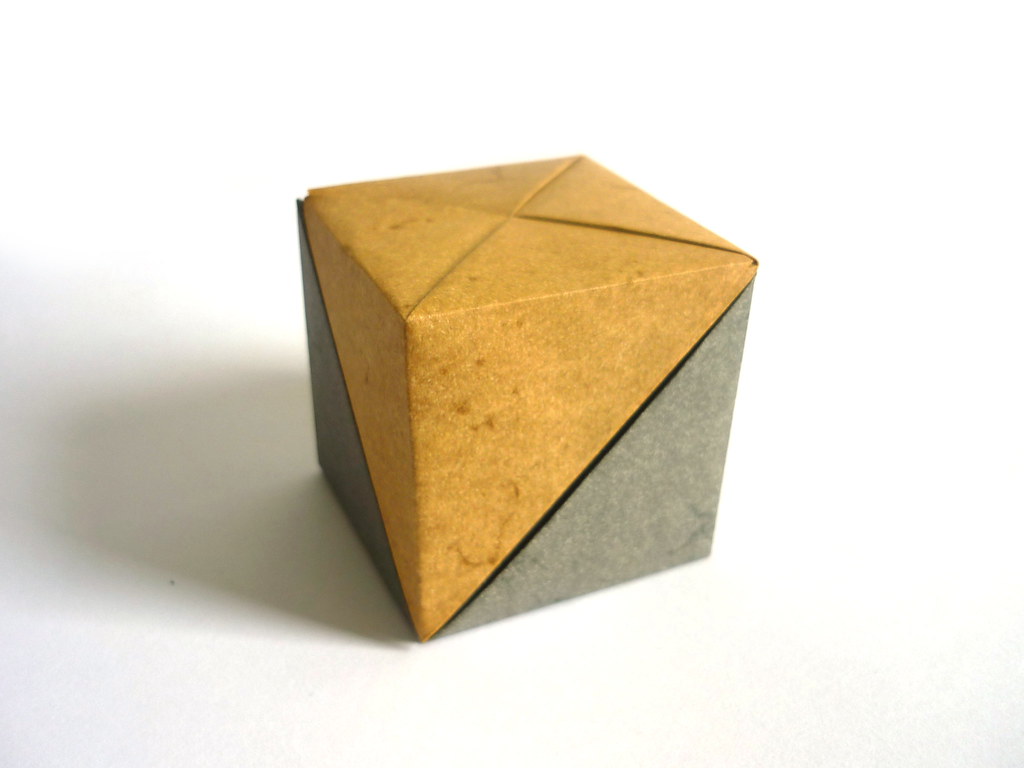
Using 12 frame pieces and 8 connectors (4 of which should be the mirror image of the others) gives this:

Finally, the face-centred cubic structure. As the name "cubic close-packed" suggests, this structure is the unit cell from stacked triangular lattices so the angles between nearest neighbours are all 60°. In this case, my work is cut out for me by Francis Ow. The cubic frame is 2/√2 times the connector length (plus the extra 1/4 of the paper width), which looks like:

And, for completeness, the CP for Francis Ow's unit is:

12 frame pieces and 36 connectors (12 mirrored relative to the other 24) come together to make:

Most importantly, the three structures together with the same original paper size look like this: